Argus Research, The Network Effect & The Cyber Safety Net. Part 1/2.
Is the Audible Sound of The Synthetic Oracle, Clinical/Safety One Auditable too? A 'Sound of Vox Doc' funf Flog (Fees log). H/t to Jurassic Carl for the eyes & Paramythia for the Greek myths.


Table of Contents Part 1/2
Introduction to Argus, Vox Doc and Dot-Com’s from a Medico POV
1.1 The Protector of IO
1.2 The Sound of Vox Doc
1.3 The Network EffecteHealth Clinical Research Opportunities Implementation Team
2.1 The Silicon Glen Endo (Co-Author)
2.2 The Billion Dollar Biller (Publisher)
2.3 The One Health Vaccine Passport (CRO)
2.4 The Embedded Eye In The Sky (CRO)
2.5 The Instant Investigator (CRO)
2.6 The 100 Eyed Researcher (CRO)eHealth Clinical Research Opportunities Beneficiaries
3.1 The Medtech Enabler3.2 The Mendelian Mentor
3.3 The Mobile Patents3.4 The Blockchain Party
3.5 The ACESSable Superhighway3.6 The Heart Switch-eroo
Conclusion and Ascension
4.1 The Cyber Safety Net and Link to Part 2/2 substack
1. Introduction to Argus, Vox Doc and Dot-Com’s from a Medico POV
1.1 The Protector of IO
In Greek mythology, Argus was the 100-eyed giant and protector of IO, the nymph who had been turned into a heifer by Zeus’s jealous wife, Hera. Argus and his 100 eyes were lulled to sleep by Zeus before slaying him on an IO rescue mission. Hera rewarded Argus for his service by placing his 100 eyes on the tail of Hera’s sacred bird, the peacock.
Argus, by it’s very definition, is a popular name with businesses and the like from the 1934 economist founded ‘deep resources’ of Argus Research Company for forcasting and informing an investment council of the same name to the ‘constantly watch’ 2006 govt funded Argus Cybersecurity Lab at South Florida University who ‘believe’ in the cyber-defensive strategy of ‘connecting the dots’ among various information sources to get to the ‘root cause’.



‘Argus Research, Inc - Clinical Evaluations’ was found in the Appendix of a clinical research ‘opportunities’ book heard quoted wayback at the source of the ‘sound of vox doc’ by a dot-com video-tech savvy, ‘Medical marketeer’ in the dawn of the internet era.:
The physicians's guide to clinical research opportunities. How to create a rewarding business & professional relationship between your medical practice and the pharmaceutical industry by Matthew D. Heller & M.D. James A. Boyle, M.D.
Publication Date: 1996’
‘Argus Research Inc’ is one of the 12 organisations in the back of the book used to expand it’s message and can be borrowed on archive.org under url physicianssguide0000hell1.
1.2 The Sound of Vox Doc
From a publicly-sourced search on the World Wide Web, Vox doc (and Vox Pop) are first clearly defined by Heartwire for Medscape in 2000 here:
Vox doc
Vox populi: [Latin] the voice of the people; popular opinion. Abbreviation: vox pop. Vox doctori: [Latin] the voice of doctors; medical opinion. Abbreviation: vox doc.
Specifically, Vox Doc was the medical opinions of doctors interviewed at the ACC Scientific Session 2000 - Medscape - Jan 01, 2000 on the subject of Sexual Activity and the Cardiovascular System. The doctors’ shared opinions refer to a presentation by Dr Popma, a VP at Medtronics today and recognises:
They (FDA) still may need some confirmatory trials to confirm what we have already seen with both types of radiation, not only to ensure that it is safe for the patients but also the operators as well.
During the time of COVID-19, in March 2021, MEDSCAPE Doctors ‘Commentary’ on COVID and the Athlete's Heart speak of when COVID first came onto the scene in 2020 and how they:
[cardiologists] were struck by the early case reports on the cardiac involvement of patients who had COVID-19 in regards to inflammatory complications, particularly myocarditis, ‘raising a lot of antennas when it first appeared’.
This medscape Commentary refers to case reports from a paper published on 03 March 2020 called ‘Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China’2 with ‘5,381 Citations’ to-date.
1.3 The Network Effect
The ‘Network effect’ is explained in ‘The history of Medscape -- The First 5 Years’3 written in 2005 from the Point of View (POV) of Peter Frishauf, a member of the original skunkworks team and CEO of SCP Communications, the former medical communications and publishing company that staffed and funded the medscape project in 1995.:
The total value of a good or service that possesses a network effect is roughly proportional to the square of the number of customers already owning that good or using that service.
Frishauf further explains that one consequence of this network effect is called ‘network externalities’ &/or ‘positive-feedback loop’ where the purchase of a good by one individual indirectly benefits others who own the good. Most importantly, Frishauf concludes:
Dot-coms operated under the belief that when a new market comes into being, which contains strong network effects, firms should care more about growing their market share than operating profitably. This was believed because market share would determine which firm can set technical and marketing standards and thus determine the basis of future competition (adapted from: Wikipedia).
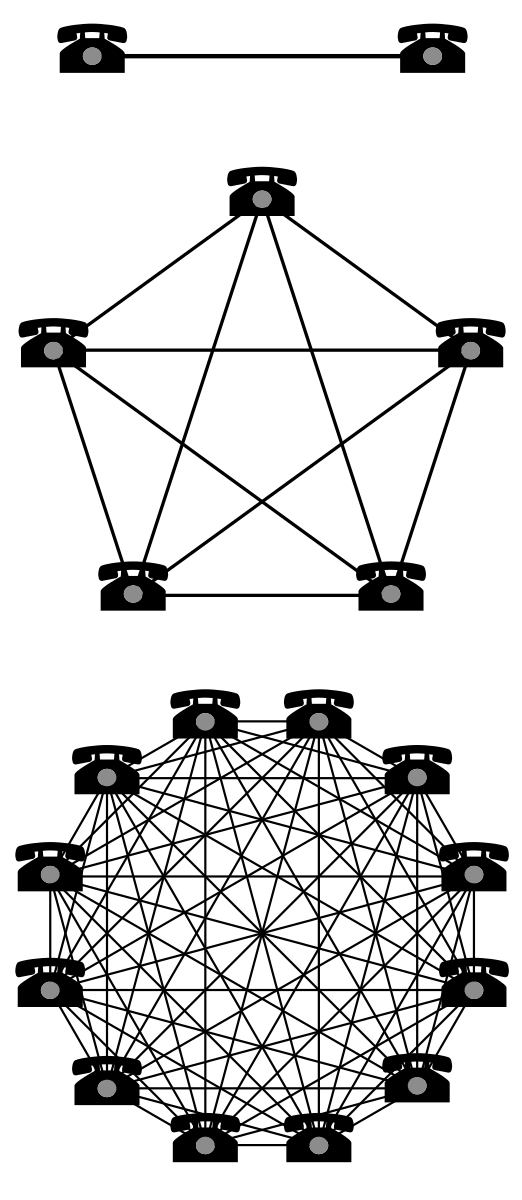
2. eHealth Clinical Research Opportunities Implementation Team
2.1 The Silicon Glen Endo
The physicians's guide to clinical research opportunities co-author is James A. Boyle M.D who has a brief biography in the introductory text on page iii. A 1967 post-doc fellowship at the National Institutes of Health (NIH) / University of California was undertaken between a medical degree and a position as a Senior Lecturer in Medicine at the University of Glasgow in Scotland. In 1973 Dr Boyle entered the pharmaceutical industry, with Pfizer, in the European Clinical Research Group in Brussels before transfering to New York HQ. The following quotes summarise the POV of this book:
[$ Incentive (Page X)]
Suffice it to say that pharmaceutical companies are prepared to amply remunerate the physician who can enlist patients into a clinical trial protocol and follow them to the completion of that protocol.[Benefit from Suffering (Page 2)]
To put it another way, the greater the potential relief for human suffering, the greater the potential market, and both humanity and the drug company benefit. If this sounds hard-hearted, realize that few others are thinking about finding, developing and marketing cures.[Invokes Declaration of Helsinki (1964) in Appendix 1 (Page 27)]
[Lay Person Consent (Page 29)]
Others hold there is no way in which a lay person can give a truly informed consent because to have a real understanding of the risks and dangers inherent in a clinical trial, that person would have to not be a layman…While it is true that physicians are in a much better position to understand the likely risk/benefit relationship of a potential new therapeutic agent, the issue becomes a question of how the doctor is going to translate these possible risks and benefits into terms that an intelligent or a not so intelligent lay person can understand.
The dramatic increase in the use of contract research organizations (CRO’s) is recognised on Page 43, and the ones who control over 40% of the market listed:
(Corning, Applied Bioscience, Quintiles, Parexel, Clintrials, and IBAH) with the names and addresses of these and other CRO’s in Appendix II.
Turning to Appendix II, found on pages 157 and 158, only Quintiles Inc is directly named from these 6 CRO’s quoted in the text, at the ‘not for profit’, IBM / ‘Dept Health, Education & Welfare’ dominated Research Triangle Park in NC along with the ‘Research Triangle Institute’.
The ‘network effect’ of a handful of these 12 listed CRO’s are logged here along with the book’s publisher. These are: ‘Service One Health Group Inc’, ‘Oxford Research International’, ‘Institute for Biological Research and Development’ and ‘Argus Research Inc’.



2.2 The Billion Dollar Biller
The Books’s Publisher ‘Practice Management Information Corp’ (PMIC) with an LA address can be found on page ii. Founded in 1989, PMIC is an independent publisher, to provide medical coding solutions to physicians, hospitals and health insurance companies worldwide. Within just a year it became the nations leading independent publisher of medical coding books. On Amazon it explains why ‘James B Davis’ and his ‘50 years’ career is known as the ‘Billion Dollar Biller’.:
His expertise in the medical coding field is the result of billing over $1 billion dollars in health insurance claims for physicans and other healthcare providers while managing one of the first online medical billing companies.
Medical Coding and Compliance Solutions, LLC (MCCS) was established in 2001 when DBL Enterprises, the creators of Flash Code merged with PMIC who then became managing partners. Flash Code, developed in 1995, was rated the #1 electronic coding software.
‘Medical coding’ is a discipline in itself. It is a digitally mandated, alphanumeric language, of which healthcare data consists of, submitted to payers for reimbursement. Fraud investigator seminars at the ‘American Academy of Professional Coders’ (AAPC) say it is all in the rules you just have to know where to find them. The AAPC as the world's largest training and credentialing organization for the business of healthcare says ‘medical coding’ requires interpretation skills:
Like a musician who interprets the written music and uses their instrument to produce what's intended, medical coding requires the ability to understand anatomy, physiology, and details of the services, and the rules and regulations of the payers to succeed.
The real ‘value’ lies in the data derived from these codes used to determine utilization, manage risk, identify resource use, build actuarial tables, and support public health and actions. The ICD (Int classification of Diseases) codes are maintained by the World Health Organisation (WHO). The AAPC recognises the discipline’s origins in:
Doctors who determined the cause of a cholera epidemic in the 18th Century from the correlating of the public ‘bills of mortality’ posted in London’.



2.3 The One Health Vaccine Passport
Service One Health Group Inc 2525 Camino del Rio South #209 San Diego
Is still at the same address and just celebrated turning 30. Today it is called A Passport Health Affiliate selling the same travel health services from the 90’s just on an app with biometric screening, your electronic health records and real time reporting. There are over 300+ clinics across North America.:
Passport Health sets the immunization industry standard.
Has leveraged its deep experience with travel medicine to service the vaccination needs of corporations, government agencies, and other large organizations.
Travel vaccinations are listed as ‘recommended’ or ‘required’ by country based on CDC and WHO guidelines. This is the same as travel clincs in the 90’s for travel to remote and exotic destinations as illustrated below, in their 1995 yellow ‘International Certificates Of Vaccination,’ from a HK clinic. Today however, everywhere is exotic and dangerous! For example, for an American to travel to deepest, dark Australia they are ‘recommending’ every vaccine from yellow fever to shingles.:
Yes, some vaccines are recommended or required for Australia. The CDC and WHO recommend the following vaccinations for Australia:



2.4 The Embedded Eye-in-the-sky
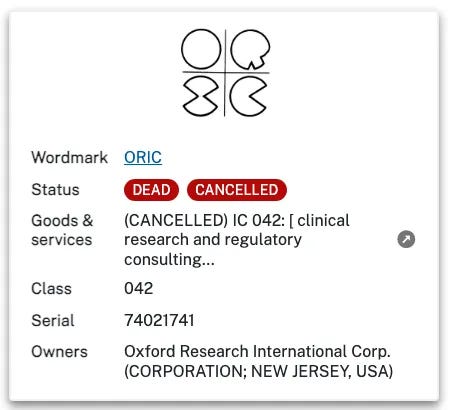
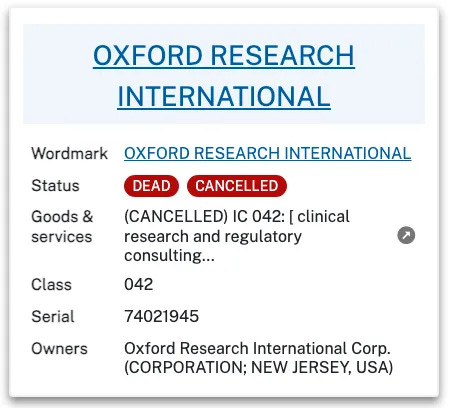
Oxford Research International 1425 Broad St. Clifton, NJ 07013
Oxford Research International and ORIC are 2 workmarks registered on 23 January 1990 by ‘Oxford Research International Corp’ (ORIC) with first use from December 1979. These are both illustrated below and registered by the same New Jersey address as in the 1996 Clinical Research book and provide Services in:
042 Clinical Research and Regulatory Consulting.
Online search pages have it listed as a ‘Radiotelephone Communications’ &/or ‘Public Relations and Communications’ company at oxfordresearch.com dba ‘Oxford Research International’ from at least 1998. On the 29 January 1996 ‘Oxford Research International (UK) Ltd’ is co-founded by 2 Oxford university residents, operating out of a company in their names called ‘Roscoe & Sahm’ from Stuttgart Germany as ‘lawyer and Social Scientest’, to carry out:
73200 - Market research and public opinion polling.
A year later, a second company ‘Oxford Research International’ is founded on 9 April 1997 by Israel Cohen on behalf of Paramount Properties (UK) Ltd before handing it over immediatley to another ‘Sahm’ to carry out:
72200 - Research and experimental development on social sciences and humanities.
Operated by a team of researchers who received their training in (Social) Research at doctoral level from the University of Oxford for select clients (1999) (2020) it uses various forms of qualitative research such as focus group discussions and in-depth interviews to precede or complement quantitative findings. Prior to ‘studying’ at Oxford, Sahm had a gap year as:
1990 - 1991 Head of International Relations @ Council of Berlin Central
An example of projects in the Media include this 2005 ‘Polling Teams Fly 'Under the Radar' for National Iraq Survey’ which they conducted five national surveys in Iraq -- two of which were commissioned by ABC News and other media partners. In this case Sahm boasts of utilising low-profile techniques with letters from the University of Oxford, Baghdad and Dohuk to pass checkpoints and ward off suspicions. Other projects promoted on it’s home page include:
On-the-ground data to drive orbital activity, utilising innovating Direct-to-Home (DTH) satellite and cable technology as a media delivery platform which reaches billions of consumers and is driven by cutting-edge technology.
Client testimonials like AsiaSat were validated in their current knowledge of the China market through estimates regarding our satellite penetration.
Reliance on AI for qualitative data and multi-variate statistical modelling for quantitative output following the principle “minds, not machines”. Findings often ‘go beyond the original remit’, especially where variables are related or ‘hermeneutic elements are connected’.
Roscoe’s publically available biographies expand on what is entailed to be a co-founder (May 1995 - 2006) of this ‘boutique’ company and an ‘oxford civilian sociologist’ on-the-ground, for USAID and US Army programs. The ethics of which are questioned at length in this 2007 article concerning Afghanistan.
A 2018 Polio Vaccine Uptake study4 from 1 of 3 studies in Sahm’s profile on researchgate: examines attitudinal and knowledge-based threats to oral polio vaccine acceptance and commitment in Somalia.:
Examined absolute levels of threat variables where being ‘unvaccinated or uncommitted’ were related to ‘multiple threat variables’ and that quelling rumors early may be important to prevent them.
Communication strategies focused on ‘enhancing trust’ in vaccinators and institutions using ‘disease facts’ that might help ‘motivate acceptance’ as well as enhancing logistics information.
Currently, according to Dun&Bradsheet, ‘Oxford Research International’ is now doing business as ‘Future Thinking’ at www.savanta.com who ‘Make Better Decisions’ for their clients sustainable future.
In the time of COVID-19, Iacuzzi, a former Oxford student, listed as Director and Secretary of the UK ‘Oxford Research International’ from 1997 to 2006, undertook a comprehensive investigation on the hot-topic of ‘Blockchain for value creation in the healthcare sector’5 as A/Professor at Udine University.






2.5 The Instant Investigator
Institute for Biological Research and Development, 2525 Campus Drive, Irvine. CA 92715
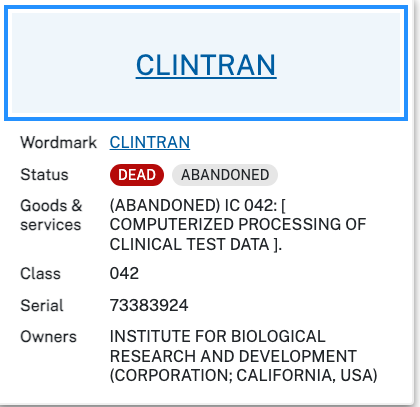
The CRO ‘Institute for Biological Research and Development’ (IBRD) Drs. Nichol, Pickering, and Bollert offered ‘a controlled system for post-marketing surveillance (PMS) of newly approved (NDA) pharmaceutical products,’ with surveillance data being ’entered into an electronic data base on site’ at least as early as 1980. This is according to wiki’s Electronic Data Capture page, as the study is not available to the lay person. Wiki also suggest the origins of Electronic Data Capture’ (EDC) may lie with this company. Their CLINTRAN wordmark for the ‘Computerized processing of clinical test data’ first use is recorded as 1981 on uspto.gov.
On Scopus Bollert, who is affiliated with Pfizer, asserts that IBRD operated as a CRO to handle investigational as well as marketed drug research programs for American and foreign pharmaceutical firms for 70 group practices averaging 30 physicians each. Bollert, acknowledges IBRD as the first CRO and from his experiences at IBRD, and his current ‘study broker’ company, writes about the regulatory process and benefits derived by participating in researching new drugs for market. Bollert’s ‘study broker’ company was founded in July 2001 for Clinical Trial Investigator Recruiting at www.invlocate.com called Investigator Location Services (ILS). As the largest ‘study broker’ in 2014, ILS described it’s market advantage in this press release:
We can literally reach hundreds of investigators in any specialty in seconds and solicit their interest in a clinical trial. No one else has this type of instant access to such a large volume of experienced investigators.
ILS celebrated 22 years of clinical investigator recruiting for Sponsors and CROs in 2023 having recruited investigators for 407+ Sponsors and CROs on 1000+ protocols. Saisfied clients include Abbott, Alcon, Amgen, Astra Zeneca, Pfizer, Merck, BMS, GSK, Covance, MDS, Parexel, PPD and Quintiles. The service is offered for free to Sponsors and CROs as ILS’s network of investigators pay the fees for help getting a specific study.:
MOTIVATED INVESTIGATORS – Clinical investigators who sign up through ILS are extra motivated to perform since they agree to pay ILS a fee when the study is launched. Therefore, our studies get a higher priority at the investigator site.
When IBRD, was sold to Toyko based Kuraya Corp in 1990, Ramzi Najm VP ‘Information Systems’ went on to lead a multi-divisional, multi-disciplinary working group for the modernization of the Clinical R&D data activities using Oracle Clinical, EDC, SAS and web-based technologies at Baxter Bioscience in 1999 and Allergan R&D in Process Innovation from 2001 to 2015.
The IBRD ‘2525 Campus Drive, Irvine’ address in Appendix II of Dr Boyles clinical research book is in a student accommodation block. Just round the corner is ‘2525 Dupont Dr, Irvine’ and HQ to Allergan. Allergan was aquired by AbbVie in May of 2020 to accelerate their efforts in delivering groundbreaking medicines around the world. AbbVie was formed in 2013 as the ‘research-based pharmaceuticals’ arm of the Abbott split with Abbot maintaining medical devices.
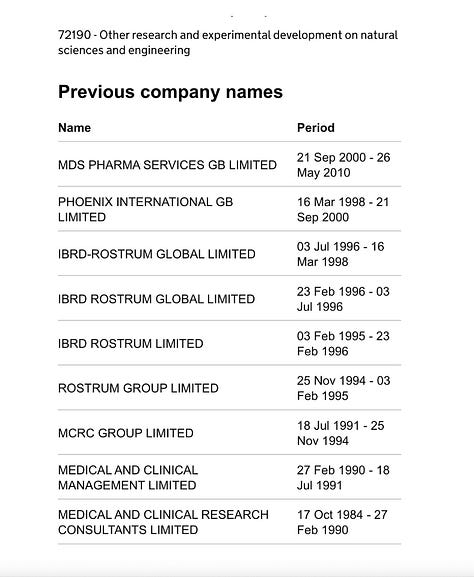
In Feb 1995, IBRD aquired UK-based CRO, Rostrum and by August 1996, IBRD-Rostrum Global had appointed Malcolm VandenBurg, ex-clinical director of Merck Sharp & Dohme in the UK, as president and CEO. The new entity added offices in South Africa, Australia and throughout Europe. Vandenburg from a 1973 degree and Honorary Lecturer in Clinical Pharmacology at St Bartholomew's Hospital in the UK has brought an incredible number of drugs to market as a Clincial Researcher. And as a registered expert witness has given medicolegal advice to courts, authorities and media in a number of high-profile cases. The company IBRD-Rostrum Global aquistion history, including Pheonix International GB are illustrated below from the UK company house record of the Global CRO, ‘Celerion GB’.:
Celerion is derived from the Latin celeritas (meaning swiftness and speed), which reflects our founding principle - that fast, reliable research is vital to a product's success.

2.6 The 100-eyed Researcher
Argus Research. Inc. 7042 E. Broadway Tucson. AZ 85710
The first (1996) Annual report of the CRO Argus Research Inc has it founded in 1991 by the Dunlap family run dermatology - wellness clinic, at the same Tucson address. By 1998 the name and ownership had changed to Hilltop -Argus Research Inc, and J James Pearce, CEO of Hilltop Research Inc and his 1,000,000 authorised share certificates and network of Directors from Ohio and California. Pearce had just purchased the ‘Future HealthCare’s operating assets. Over the next decade, Hill Top Research was recognised as one of the world’s leading clinical research companies conducting efficacy and safety studies for the personal healthcare industry and also provided clinical trial services in Dermatology Rx with the dual goal of:
Providing sponsors with a single, global research network [and] standard setting pioneer in dermatology research.
The 2000 to 2005 annual reports of ‘Hilltop-Argus Research Inc’, have ‘Radiant Research Inc’ in Washington State listed as investing in and directing the company at the same Tucson address. In 2006 these change again to ‘Covance cru Inc’ directors at the research/science focused Carnegie Center address in Princeton New Jersey and their last annual report was submitted in 2009. CenterWatch reports at the time that Radiant Research sold 8 phase I units to Covance for $65M and generated $25M in annual revenue hiking Covances pharmacology bed capacity.
From a 2013 history, Covance was created at the end of 1996 as a spinoff of ‘Corning Inc’, had offices in 17 countries, ranked 2nd in size as a CRO in the US for the worldwide pharmaceutical, biotechnology, and medical device industries [and] provided ‘health economics and outcomes services’. In 2014 labcorp acquired Covance for Approximately $5.6B, creating the:
World’s Leading Healthcare Diagnostics Company.
In the time of COVID-19, Labcorp announced on 28 May 2020, that their drug development business, Covance, through an alliance with Medable, got to:
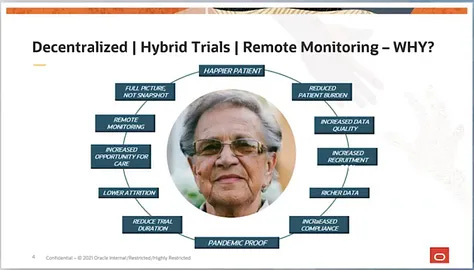
Expand their technology ecosystem to accelerate the adoption of DeCentralized clinical trials, often referred to as hybrid and virtual clinical trials creating the first data-driven, decentralized trial ecosystem.
In late 2019, Covance had unveiled how decentralisation was possible through Labcorp’s conveniently located resources like Walgreens etc. The trial hub site ‘www.covance.com/virtualtrials’ shared in the announcement can be viewed in this redirected 19 April 2020 archive that defines DCT’s as follows:
A highly patient-centric approach to study design and execution [that] blends a global patient support ecosystem with technology to allow patients to participate in clinical research from virtually anywhere.
Meanwhile 2 days later, on 21st April 2020, LabCorp’s COVID-19 self-collection test kit receives FDA Emergency Use Authorization permitting nasal swab specimens to be collected at home using it’s Pixel by LabCorp™ [test kit] if recommended by a healthcare provider after completing a covid-19 questionnaire ondemand.lapcorp.com:
AVAILABLE FOR AGES 2+ Kid-Friendly COVID-19 Testing When your child or teenager needs to get a PCR test for travel, summer activities, or possible infection, Pixel makes it easy.
By January 2021 a Covance ‘virtual trials’ sister hub ‘clinical testing’, at the same site with the title ‘Cell and Gene Therapy Solutions Education Center’ is promising pharma:
With specialized expertise, coordinated capabilities and focused investments across pre-clinical, clinical and post-approval phases, we’ll help you to reduce the time and risk in your cell and gene therapy product’s development.
In March 2021, Covance becoming a ‘fully decentralised CRO’ is promoted at Oracle Health Sciences (OHS) and discussed in their ‘The Latest Dose’ podcast re driving the implementation of DCT’s globally.






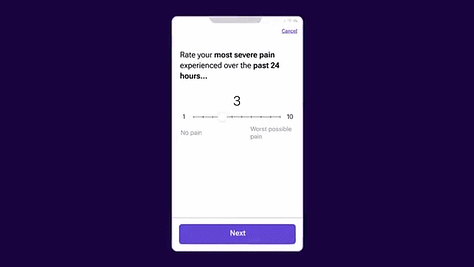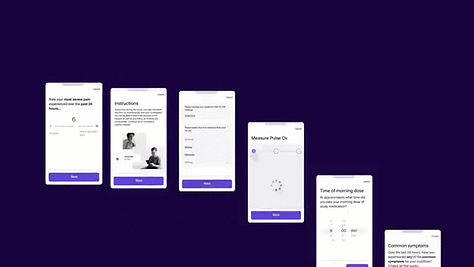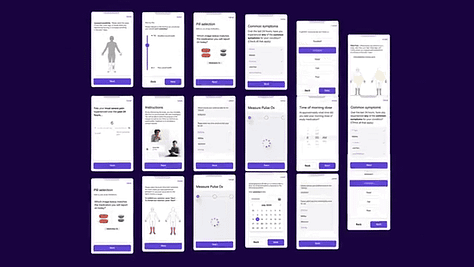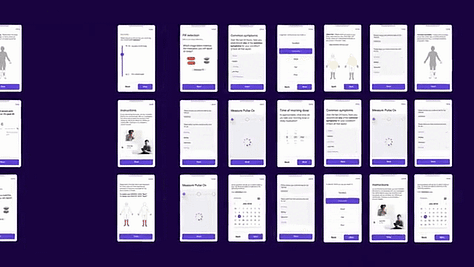

3. eHealth Clinical Research Opportunities Beneficiaries
3.1 The MedTech Enabler
This Covance/Medable DCentralised clinical Trials Alliance majorily boosted both patients AND therefore business for Medable. Over the course of the pandemic, Medable disseminated many clinical trials, including the Covid-19 vaccine and therapeutic therapy, to more than one million people across 41 countries and in more than 60 languages. Medable CEO and Co-Founder said in a November 2021 CNBC Interview:
There was a lot of interest but not a lot of uptake, so when Covid hit, suddenly, the entire medical infrastructure on the research side needed a technology like Medable to be able to execute clinical trials.. within a week I was getting calls from all over the world.
For the year 2021 on linkedin, ‘Medable Internet’ makes it to their no# 22 spot of the Top 50 US Startups on the rise in the wake of the pandemic. Most common job titles at Medable are reported as Digital or Technical Project Manager and Product Manager. What the ‘Medable Internet’ company does is reported as:
Medable is a global platform aiming to get effective therapies to patients quickly, minimizing the need for in-person clinical visits. By enabling digital clinical trials, the company accelerates the development of vaccines and treatments — including those for COVID-19.
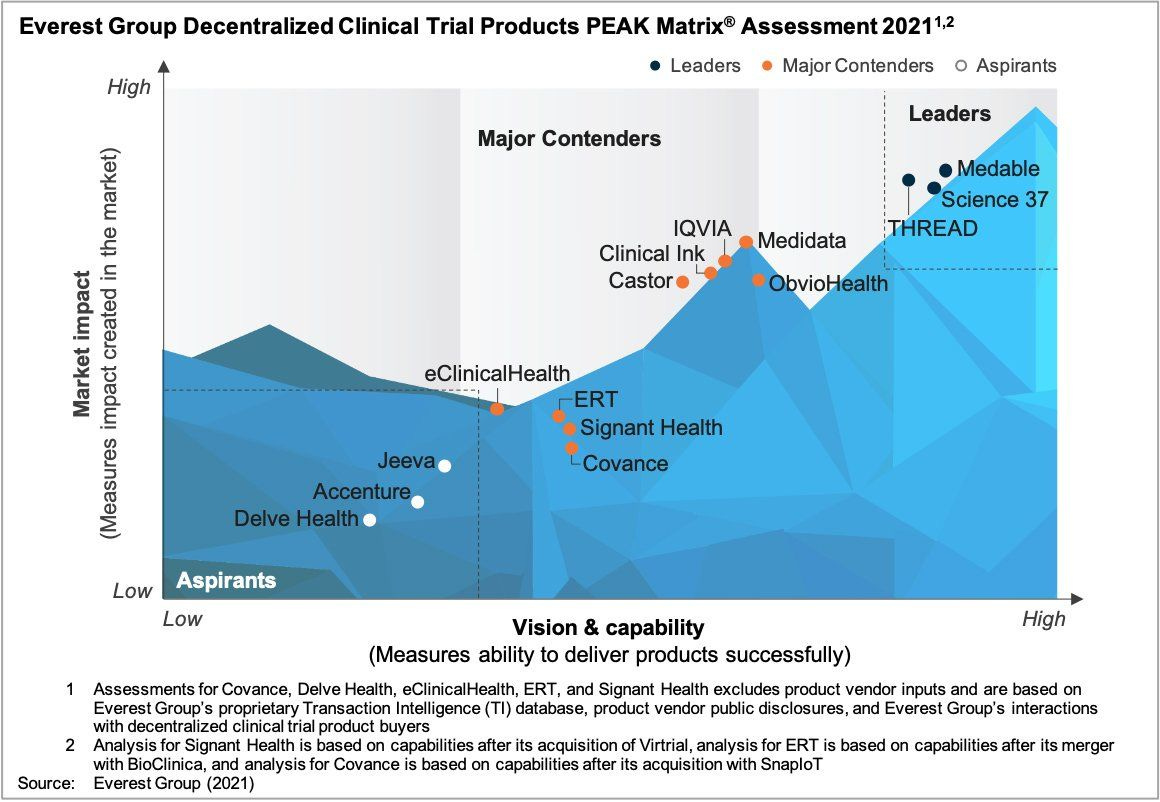
Everest Group is also thanked publically on Linkedin, by Medable’s VP for Sales for recognising Medable’s Peak place in their DCT Products assessment of Market impact versus successful product delivery for 2021:
2025 Medable.com offers ‘Medable Decentralized Clinical Trial Software (DCT and electronic Clincal Outcome Assessment (eCOA) solutions’6, Featuring accelerated to ‘real time’, infinite patients ‘at scale’ and HIPAA compliant DCT’s sold as an oxymoron of a ‘D-Centralised’ infrastructure ecosystem in the cloud (Mobile health, Software-as-a-Service) on a ‘Centralised’ ‘Clinical/Safety One’ ORACLE Enterprise platform Solution.
The recent medable marketing video shared publically by Canadian James Marcel Sas, the ‘Chief Architect’ and Co-founder of Medable promises to ‘Cut trial timelines by >50% using one platform, from start to finish’. This advert provided the source of the medable gifs used in the ‘image gallery’ above. Sas previously won a team award in 2005 for ‘Best Interactive (Gemini)’ Team with Cornergas.com for Comic Genius.
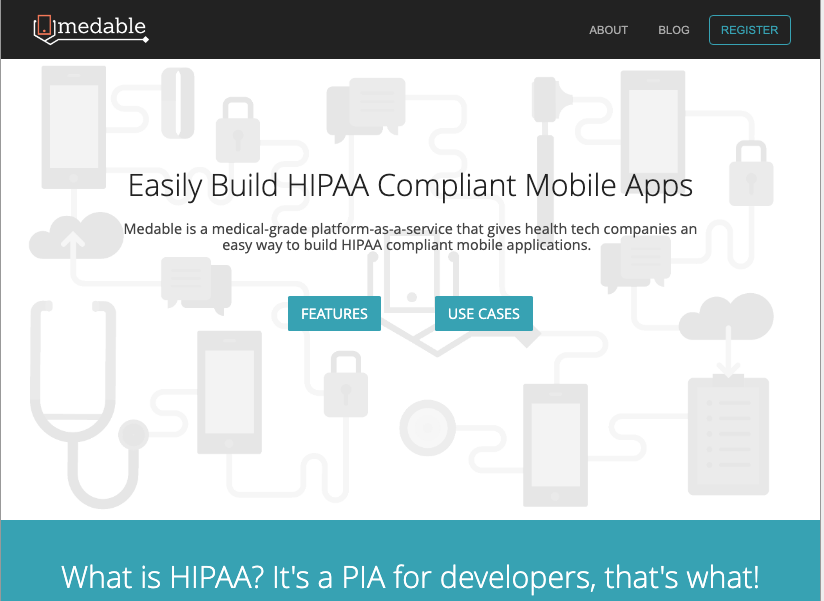
What the exact same site was promoting at the onset of Medable in 2014, www.Medable.com goes to the very core of what medable is:
What is HIPAA?
It's a PIA for developers, that's what!
The Health Insurance Portability and Accountability Act (HIPAA) is the principal set of regulations governing the storage and acceptable use of personally identifiable medical data.
HIPAA was passed in 1996 at the dawn of the Internet era.
Basically, it's a huge PIA to develop health tech apps that are 'HIPAA compliant'.Enter Medable.
Medable's medical-grade, mobile-first platform was built with HIPAA compliance at its core.
In addition, Medable is designed for interoperability, and makes it easy to meet Consolidated Clinical Document Architecture standards.
Medable data is portable, actionable, and its real-time rich-data relay enables wearables, implantables, and in-home devices to be a part of the care conversation.
Is it a play on words? Where PIA = Pain In the Ass and Patient Information Assistant medical app or even Privacy Impact Assessment?
In 2024, on the 14th February, Rob Coker in the ‘The Medicine Maker’ (RC) questions whether to Drop the 'D' from the DCT now the pandemic years are over and deduced from asking the experts, who are medable of course:
There was consensus that the 'D' will eventually be omitted from the DCT acronym altogether as DeCentralization becomes the industry standard.
Digital technology enables trial sponsors to 'cast their nets' further than ever before, inviting and including participants from more remote areas, as well as those from 'underrepresented' cultural backgrounds.
In April 2024, H1 ‘Towards Healthcare’, talk on ‘Two Life Science Entrepreneurs Unveil AI’s Future in Clinical Trials’ at 13:33, Dr Longmire is asked somewhat incredously:
Can you explain to people uh a decentralized clinical trial? and also explain to me how you predicted the future that that was going to be like a thing you basically created a category uh basically for pharma companies? and I I would love to know if you knew it was going to be this big?
Four years before, in January 2020, this GoodStory transcript ’Ep. 28 — A dermatologist from a pedigreed Los Alamos science family fights to break the mold in sports, medicine, and Silicon Valley’ answers H1’s future questions as to what Dr Longmire and her epigenics research network needed:
To understand the epigenome.. we needed a capability of capturing data from patients in their everyday life, very similar to type of data on your mobile device, location, weather, the various factors you may be exposed to on a daily basis and we need to help connect with patients worldwide.
Hence the dual mission of Medable:
To revolutionize Clinical Trials (CT’s) using a ‘platform + network’ approach by 'uberizing' them and opening the door to have access & actively improving diversity by:
Screening of patients for genetic variants out in their everyday life, identifying those gene variants and getting them into the personalized medicine clinical trials to determine if those interventions are effective for patients.
This direct interaction between patients and doctors conducting these trials ‘without the middlemen’ also equates to improving costs.
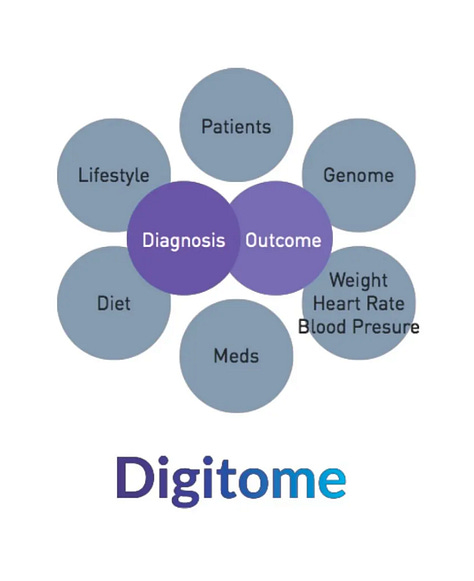
A Data-driven effort to populate Medable's Digitome defined as
A digital representation of human health and disease that is a data common across all of the Medable users and clients & partners.
For example, if the Digitome has a study in rheumatoid arthritis for a particular partner & another partner study in psoriasis, their researchers can combine data sets to understand the overlap of disease and drive their deeper understanding of medical conditions.
3.2 The Mendelian Mentor
Dr Longmire is a ‘Stanford-trained physician-entrepreneur’ and epigeneticist who researched aging and rare diseases by studying the different health outcomes of identical twins during a Dr Howard Chang residency. For a school science project, Dr Longmire undertook four generations of hamster breeding to observe ‘Mendelian Inheritance’ with her father and mentor Jon L Longmire. Jon L Longmire is an expert in recombinant DNA technology, was part of the ‘Human Genome Project’ at Los Alamos National Labs (LANL) and ran rare bird breeding programs with WWF certifcation from a PhD backround in developing an assay to test the sex of birds so that they could be bred in captivity.


Dr Longmire also learned that through a dedicated team of experts, you really can solve problems that seemed impossible to solve beforehand from her grandfather, Conrad Longmire. Conrad Longmire was a nuclear physicst and expert in ‘electromagnetic pulse theory’ who was part of the fifities Los Alamos ‘secret community’ that developed the hydrogen bomb.7
A 2008 ‘Biographical Sketch’8 of Jon L Longmire expands on his role as Group Leader for the Biosecurity and Public Health Group (previously the Molecular Microbiology and Immunology Group) at LANL:
Provided oversight for approximately 60 scientists and technicians and managed a wide array of biological programs and biosafety laboratories, including the development of a new, state of the art BSL-3 laboratory.
Specialties: Research and development, program development, transition of scientific R&D to private sector.
The 2025 Jon L Longmire up-to-date profile9 in ‘recent retirement’ expands on previous assignments ‘relevant to national security’ including time at the DTRA-ASCO (Defense Threat Reduction Agency’s Advanced Systems and Concepts Office). Experience in several areas of biological research, include: genomics, microbiology, molecular cloning, conservation genetics, biosecurity and biothreat assessment. Membership of 3 ‘Interest Groups’ are also publically profiled. These are:
‘Pandemics Bioterrorism & Global Health Security’
The Group’s Manager (who created a timely gap analysis for a 6-hospital system on disease preparedness in Tuscon Arizona and coordinated the COVID-19 Netflix response) describes the groups purpose in their biodefense hub here and the Group owner ran a topical pre-covid-19 summer workshop in July 2019 under the same name.‘The Academy of Infectious Diseases (AIM) Group’
The Owner of the Group is profiled here with a background in Wyeth Pharma Inc and the more recent relationship to both the UK companies, AIM and Vox Marketing, dissolved in October 2020; and

In the time of COVID-19 in October 2021, Jon L Longmire acknowledges Medable for starting a new self-care technologies conversation with insurers:
At present, it costs 5x more to develop a cell / gene therapy vs a traditional therapy. Are insurers & health systems ready to support new self-care technologies & recognize prevention as an integral element of service provision & cost reduction? (Medable)
3.3 The Mobile Patents
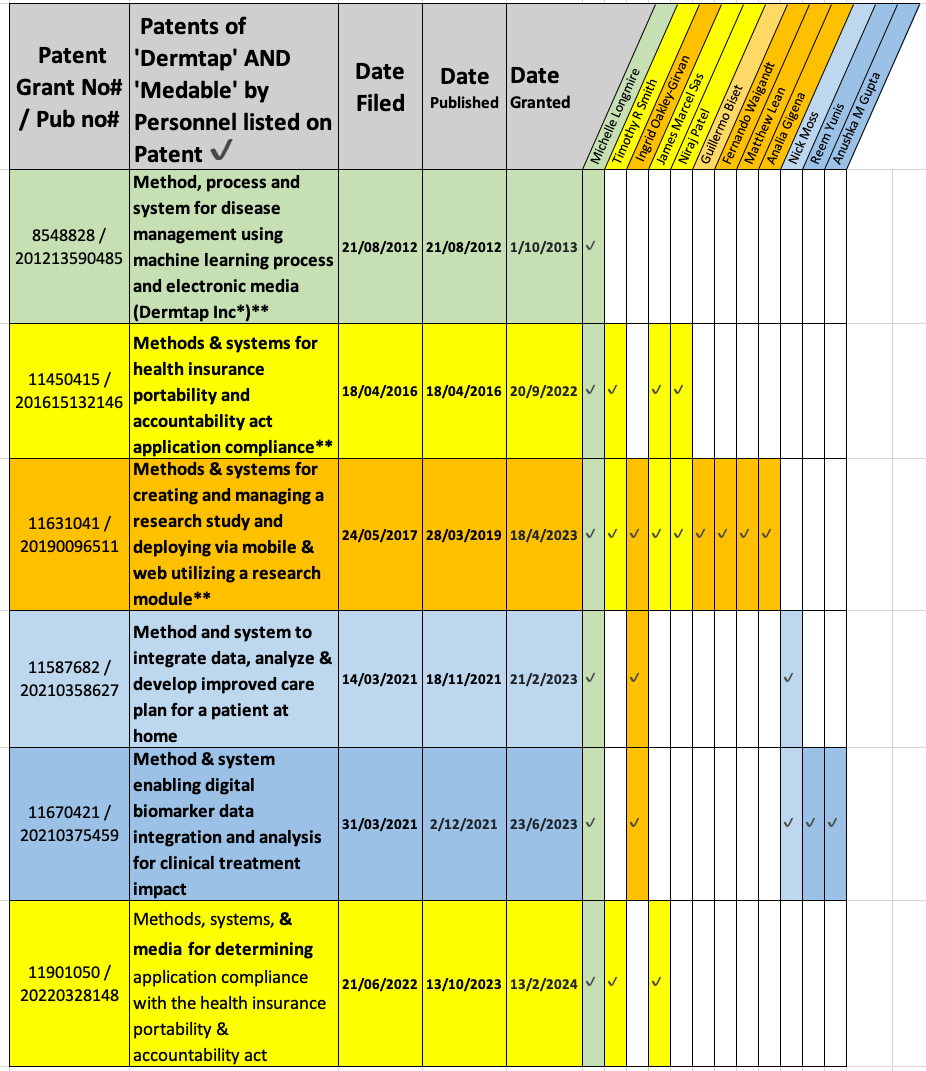
Back in 2012, Dr Longmire through Dermtap (previous name of Medable), filed the first of 6 patents with the title 'Method, process and system for disease management using machine learning process and electronic media’. Claims include:
Registering a user to use a data management system to obtain a treatment for a disease using an electronic mobile device; and
The medical history of the user is stored in a database with secure HIPAA compliant and encryption controlled set of rules.
The Medable mobile phone patents are summarised by filing date and highlighted by the Medable Personnel listed on the Patent in the table below.
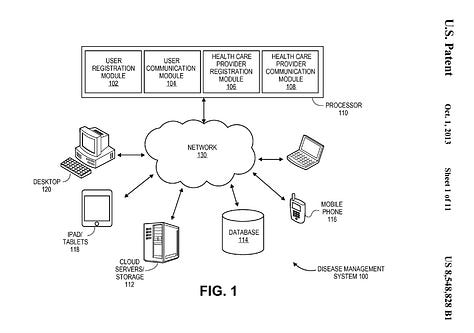
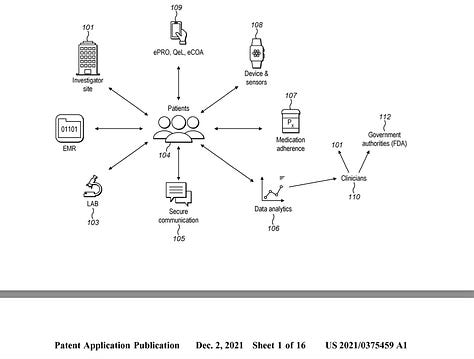
This 2011 dermtap ‘centralised’ cloud-network-focused design is illustrated next to the 2021 medable ‘decentralised’ patient-focused-design. This later design supports digital biomarker cluster-head responses in both interactive and streaming modes for biomarker calculations.:
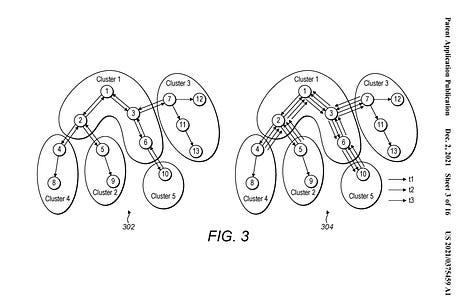
Within the dermtap patent under ‘Other references’ is the paper:10
Liu, L., & Liu, J. (2011). Mobile phone-enabled control of medical care and handicapped assistance. Expert Review of Medical Devices.
Jing Liu, in the ‘Recent Application in Biometrics’ also co-authored the 2011 ‘Biometrics on Mobile Phone’,11 at the Dept of Biomedical Engineering, School of Medicine in Tsinghua University P.R. China’. This research acknowledges under section 5:
This work was partially supported by the National “863” Program of China, the Tsinghua- Yue-Yuen Medical Sciences Fund and the Funding of the National Lab for Information Science and Technology at Tsinghua University.
Jing Liu’s biometric lab also developed the ‘Mobile Health Examination Launched on the Phone’ (M-HELP)12 for a timely health examination for incipient disease detection and prevention and to address technical barriers. This was based on the design of a Google Android but with the development of different wireless biomedical sensor modules where a mobile phone was incorporated into a central terminal for personal health examination.


‘M-HELP’ named phone &/or app programs are given out in many conflict / migrant areas with the promise of free health. For example in April 2016 an M-HELP mobile app was launched in Kazan to help and adapt migrants with the support of the All-Russian social movement. And in 2022, a company called m-help.com, partnered with the Ministry of Health of the Republic of Latvia in the international medical aid project, within the framework of which Ukrainian civilians who have suffered in the war had the opportunity to apply for free treatment there.:
The M-Help team organizes the transfer, maintains the Data System and monitors the daily needs of patients in Latvia.
3.4 The Blockchain Party
In 2015, through the Medable CEO’s time as a Stanford faculty member, the first $700k was raised and the decentralized capability — got the attention of entrepreneur Bob Duggan and his $3M. Duggan’s stint on the board is brief along with fellow colleague Dr. Zanganeh from Pharamcyclics. The pair are responsible for the game-changing treatment, ibrutinib, for a wide range of blood cancers through collaboration with Johnson & Johnson in 2011. This was Sold in 2015 for $21B to AbbVie Inc, it was considered the largest biopharma sale ever at the time.
All the ‘Form D’ submissions by date, $ amount, ‘seat on the board’ and investor series are highlighted in the summary table below.
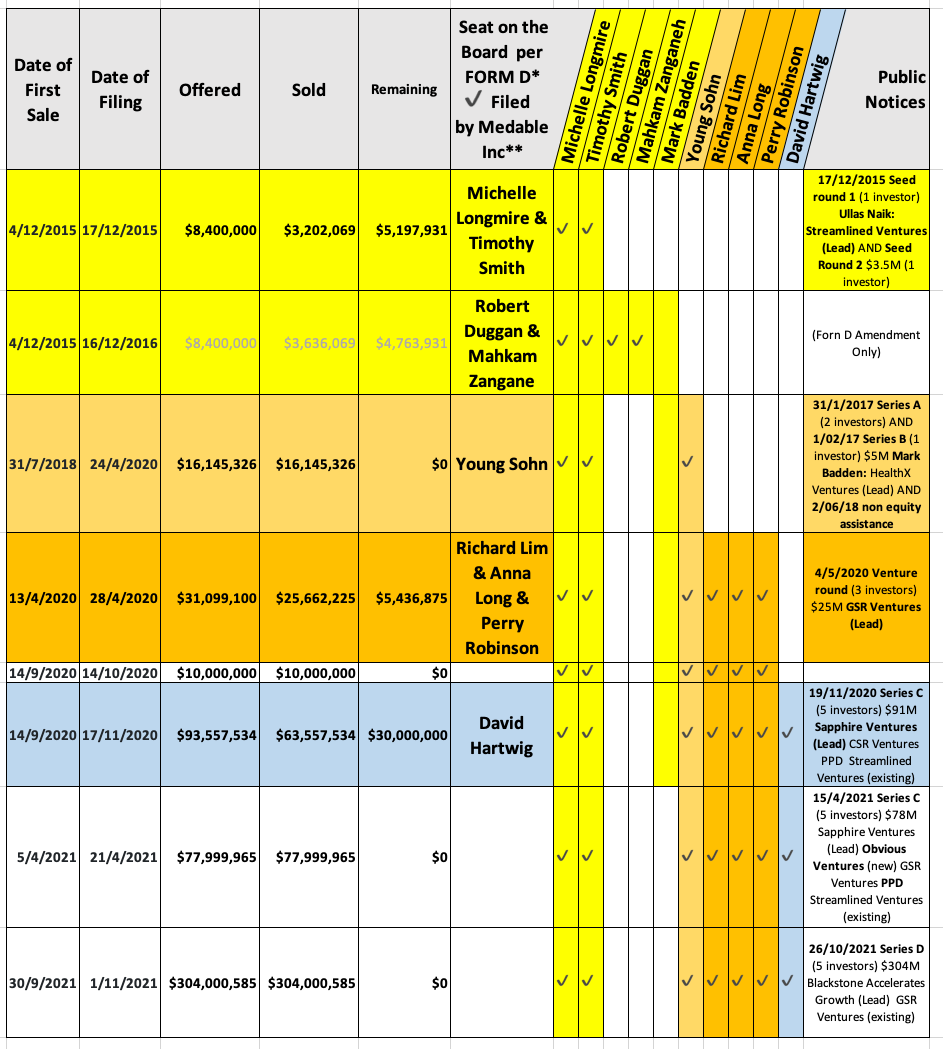
In 2017/2018 Medable launched it’s Series A and B funding rounds. ‘Young Sohn’ comes on the board of Medable and MedaSystems at this time. Sohn spent the first six years at ‘Oracle Corporation’ in the pharmaceutical vertical organization before founding life science software companies Nomadic, then Veeva (working with Merck and pfizer to streamline their clinical trial process) and Vlocity, a cloud based software app company. Like all successful startups these were eventually sold for a lot of money, to the likes of Salesforce, earning a place on the Forbes ‘American self-made Women’ in 2024 with a Net Worth of $550M.
Just in time for COVID-19, Medable’s Senior VP for Enterprise Sales kicks off a big week at the 38th Annual JP Morgan Healthcare Conference on 13th January 2020 with an event and AfterParty:
‘Medable Dcentralise 2020’.. The event begins with the College Football National Championship at 4:30 PM and the debut of our new Medable T-Shirts celebrating our China expansion and Tigers football game. Food and drinks, throughout.
According to Trends from ‘Syneos Health’ the ‘38th Annual JP Morgan Healthcare conference’, is an annual ritual in healthcare in San Francisco and showcased China, Biotech, Digital Medicine and Medtech with 9,000 industry leaders - corporate executives, bankers, analysts, investors and journalists.
Richard Lim comes on the Medable board from April 2020 Venture Round and explains in a Vator News interview13 on 17th June 2020, that the key to investment is knowing your industry and the people in it, using Medable as an example:
We recently made a relatively limited investment for us in a company called Medable involved with virtual clinical trials.
When COVID-19 hit, we were truly fortunate in that it totally turbocharged their business because suddenly the large pharmaceutical organizations that had been looking at this technology, whether it was AstraZeneca or early adopters, the coronavirus basically made it a necessity for them.
Lim’s industry is ‘Digital Health’ and has been focused on AI since 2016, with his venture Capital Firm, GSR Ventures that has a US office in Pal Alto. Lim has an MBBS degree (MD equivalent) from the National University of Singapore and an MBA from Stanford University in computer science and business. Prior to GSR Ventures [2004], Lim was a serial entrepreneur and executive at Lotus Development Corp. and the National University Hospital of Singapore focusing on investments in early-stage companies that apply artificial intelligence to healthcare and enterprise sectors. Lim has been waiting for the ‘ right time’ with his ‘AI insights’ for this ‘Medable’ opportunity for his whole life:
[After] Medical school.. had the intention of working in health tech technology, graduated from Stanford in 1988.. apparent to me that the healthcare industry was not ready to deal with DOS-based PCs with 18×24 character displays.
Gratified.. to see an article in Forbes [say] that the first trillionaire will be some entrepreneur who applied AI to healthcare. A trillionaire founder implies that the company is going to be worth $4 or $5 trillion. That’s a big number.
In 2025 according to Pan Alto’s Traxnn ‘Technology + Human-in-the-Loop for Deal Discovery’:
GSR Ventures is a venture capital firm founded in 2004.
It is primarily based out of Beijing, China.
Its investments are spread across a wide range of sectors from High Tech to Enterprise Applications and Blockchain Technology.18 partners in US and China
Partner Locations: Singapore, San Francisco, Beijing, Chaoyang and Stanford.



Anna Long is also listed as arriving on the board of Medable in the April 2020 Venture Round. AL was Senior VP of Strategy at PPD at the time. Thermo Fisher Scientific aquired PDD on Apr 15, 2021 at an acquisition amount of $17.4B according to this Tracxn report. PDD as Medable’s preferred CRO partner collaborated on nearly 70 digital and decentralized trials during COVID-19. Long’s colleague was excited in this 24th June 2021 BusinessWire:
As Medable’s preferred CRO partner, PPD is excited for the arrival of a complete SaaS platform and the associated training and certification program.
Supports partners to codify emerging skills and learning paths that empower individuals and teams to design, build, deploy and support decentralized and hybrid trials.
In August 12, 2023, the Medical Buyer reports that the PPD clinical research business of Thermo Fisher Scientific Inc., has been granted a five-year award for NIAD under cooperative agreement U01AI178756 to ‘move the science of transplantation forward’ to provide a:
Transplantation Statistical and Clinical Coordinating Center.
The November 2020 and April 2021 Medable Series C funding rounds has David Hartwig arriving on the board as Lead Investor with his company Sapphire Ventures. Dr Longmire is excited to be featured in their first AI-native market map defined as:
AI-Native: leveraging AI as a core capability to unlock entirely new use cases and capabilities. [In Medables case] in healthcare and specifically in drug development.
Sapphire’s announcement on the 19th Nov 2020 mentions their ‘earlier healthcare technology investments’ as Fitbit, 23andMe and Livongo. Hart is also listed on the board and advisory roles of 7 other companies including ‘Phoenix Labs’, a Canadian ‘Massively’ multiplayer Role Playing Games (RPG) company founded in 2014 by former RIOT Games developers. At the time of Sapphire investment in 2018 ‘Dauntless’ had surpassed 2M players globally running around in a ‘fantasy world’ hunting massive Behmouths. Hart pledged that Phoenix Labs has ‘ambitious plans for 2019 that will shake up the online world in new and exciting ways.’
In the final October 2021 $302M Series D round led by Blackstone (the world’s largest alternative asset manager, with more than $1 trillion in AUM) crunchbase reports:
[Medable] will use the new cash to accelerate market adoption of digital and decentralized clinical trials that its platform enables.
3.5 The ACESSable SuperHighway
Back in 2012, Tim Smith, a web engineer and a co-founder of Medable, filed for the wordmark ‘Dermtap’, and by 2013, Dr Longmire was able to go on stage at the University of Southern California (USC) ‘Body Computing Conference’ (USCBCC) to demonstrate and ‘beta trial’ the Dermtap ‘Safe and secure patient-centered collaboration in the palm of your hand’ app:
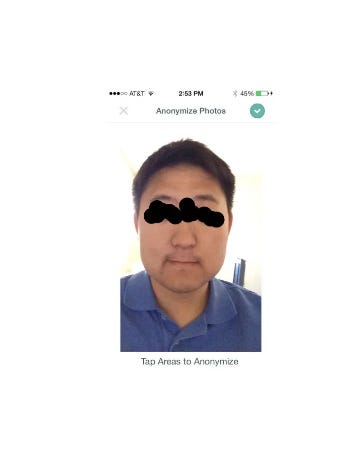
Dermtap is a fully HIPAA-compliant mobile app for secure and safe image storing and sharing. With Dermtap, Dr. Longmire’s primary goal is to make “digital doctoring” a reality, enabling digital communication between patients and providers.
This iMedicalApps article explains all the ‘exciting’ interactive features like the ‘tap to anonymise’ image feature. This 2012 Dermtap wordmark and Medable’s 5 registered wordmarks to-date and more recent pending electronic consent wordmarks are illustrated in the 2 tables below.
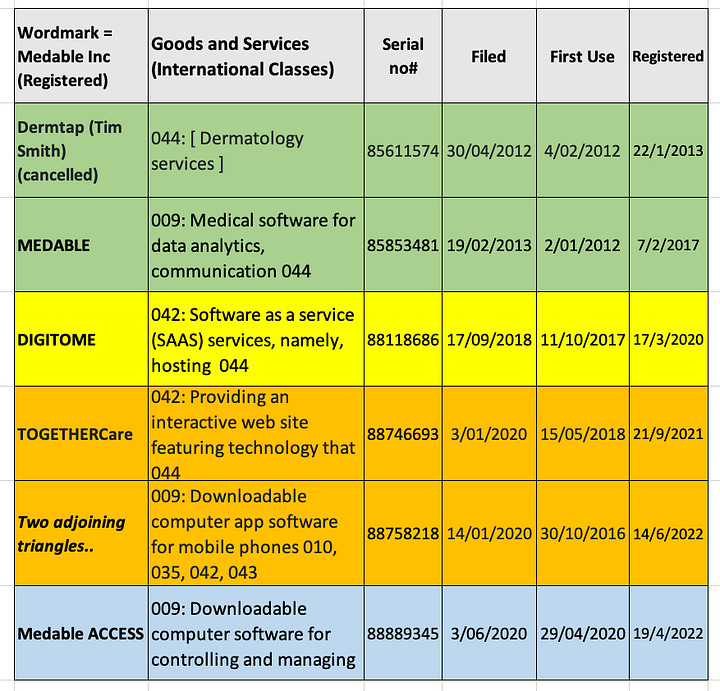
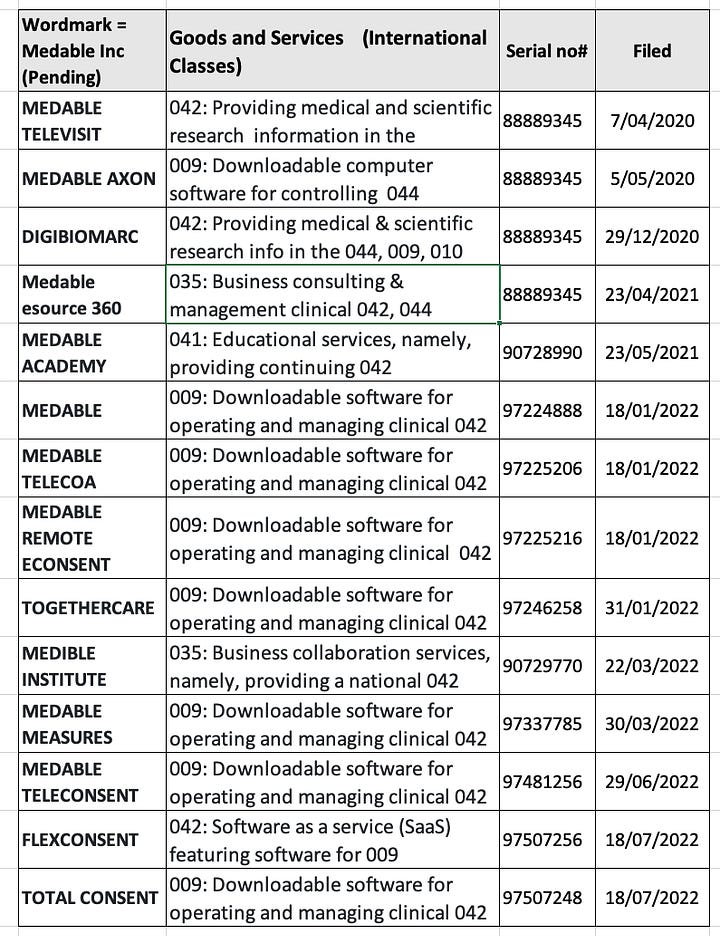
In the 2018 Digitome wordmark application on uspto there are 30 odd pages of presentation (Researchkit as a Health Data Platform). In the 2018 mHealth forum Medable were an ‘Innovation Partner’ and in the preceeding 2017 mHealth for Clinical Trials, Medable were an ‘Expertise Partner’ side by side with ‘Oracle Health Sciences’ (OHS). Both Dr Longmire representing Medable and Jonathan Palmer representing OHS spoke on the same day with Pfizer speakers and the like on the same ‘exploding’ topic. Prior to Palmer’s 19 years in clinical teams at OHS he worked within data management and clinical IT at Quintiles and Parexel, and IBM.:
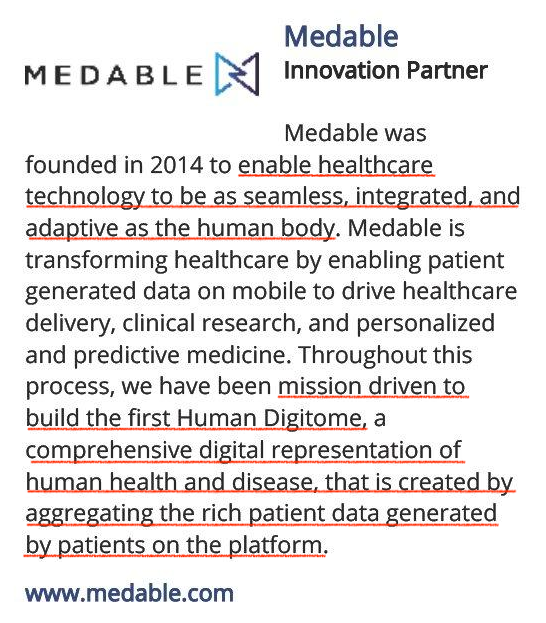

The 2018 digitome promotional material features 1 of the 2 Medium pieces by Dr Longmire written in 2017 ‘From clinical care to continuous care’14 on the Medable product, Synapse, being part of Apples new carekit solution that:
Allows healthcare organizations to leverage CareKit by handling their complex technology issues — like HIPAA compliance and medical record interoperability securely and at scale with analytics capabilities.
The medium post concludes with:
Soon analytics and machine learning will provide insights back to patients directly, alerting to high risk behaviors and providing guidance to optimize outcomes. Whether it is a doctor or an algorithm, mobile closes the care gap and creates critical connectivity for continuous healthcare.. in our daily lives.. personalized, and that can leverage analytics to identify disease and complications earlier to optimize outcomes.
What happened to this 2018 Medable Inc Introduces Blockchain for Healthcare on page 109 in the Digitome wordmark application? If this holds true surely Medable ‘enables [an] auditable’ data trail too?:
INSIGHT network: a blockchain powered data exchange that aligns incentives among all stakeholders & enables auditable, transparent, and self-directed data sharing of digitome data.
As researchers use the Medable platform, they are able to contribute data to the digitome in exchange for funding and other research resources.
Access to the dataset can then be requested by third parties via Smart Contracts, which utilize self-sovereign digital participant identities to give individual participants the power to consent and be rewarded for sharing the requested research data and/or consideration of a related clinical trial.
INSIGHT enables at-will data contribution so that each participant can donate data to specific research efforts.
In the 2020, 141 page ‘TogetherCare’ wordmark application there is an Invention Disclosure Report on page 3 that has Dr Longmire as the Principal Investigator (PI). The other research personnel match the names from the Medable ‘mobile research study’ patent filed in 2017. The Nature of this Invention Disclosure is:
TOGETHERCare is a mobile application on a smart software system that informal caregivers will use to develop and implement home-based care for cancer survivors.
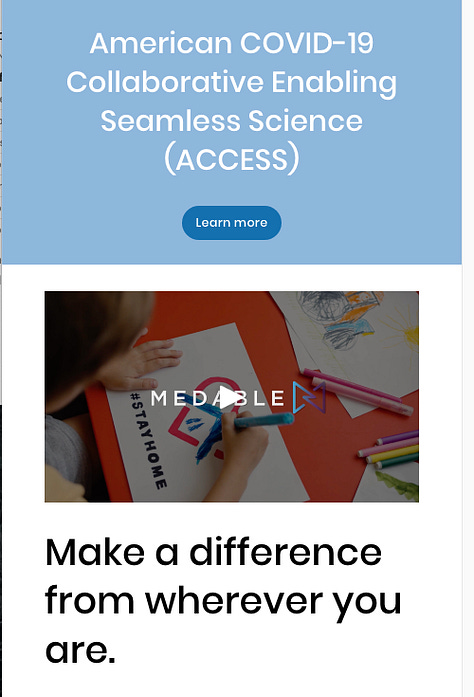
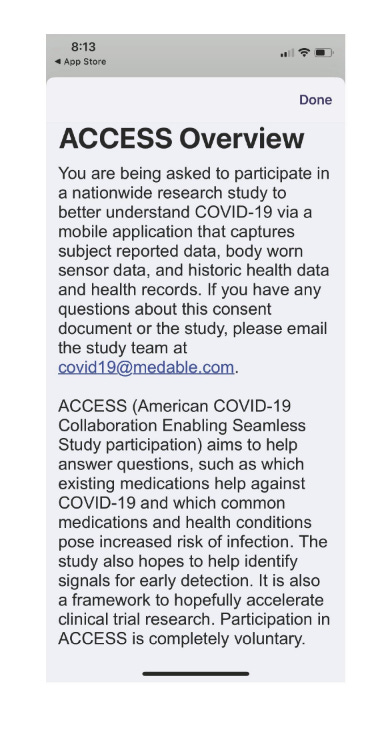
On April 20th 2020 all systems are go for Medable with the launch of their ACCESS superhighway:
ACCESS American COVID-19 Collaborative Enabling Seamless Science:
Datavant tokenization for medical record aggregation across siloed sources WITH BioIntelliSense, Inc,, FDA-cleared [Biosticker] remote patient monitoring WITH PWNHealth molecular and antibody COVID-19 testing WITH
Medable dynamic direct-to-patient trial participation and secure personalized matching to qualified research AND
Parexel [developing innovative new therapies with client partners] AND
American Heart Association shared vision to accelerate the end of COVID-19.
This article in the Biotech Bay of Biospace covers the launch in more detail, the illustrations below are sourced from the access.medable.com site archive. A Global Televisit capability was also announced.

This piece by Micky Meece interviews Dr Maut, CEO of BioIntelliSense's Data-as-a-Service (DaaS) platform at the time:
Now, in the course of 24 hours, instead of two measurements of temperature, we have 1,140 measurements of temperature, plus heart rate, plus respiratory rate, plus how often you're coughing.
Tremendous surge of requests for the BioSticker in order to monitor people, especially in mission-critical settings like nuclear power plants.
Page 1 , 2 & 5 of the ACCESS app Specimen from the uspto wordmark application says:
The study also hopes to help identify signals for early detection. It is also a framework to hopefully accelerate clinical trial research.
We also want to learn how COVID-19 is spreading throughout the United States and find ways to predict and prevent further infections.
It is important to note that we will not provide any information TO YOU about your health or clinical interpretation of your data from the study.
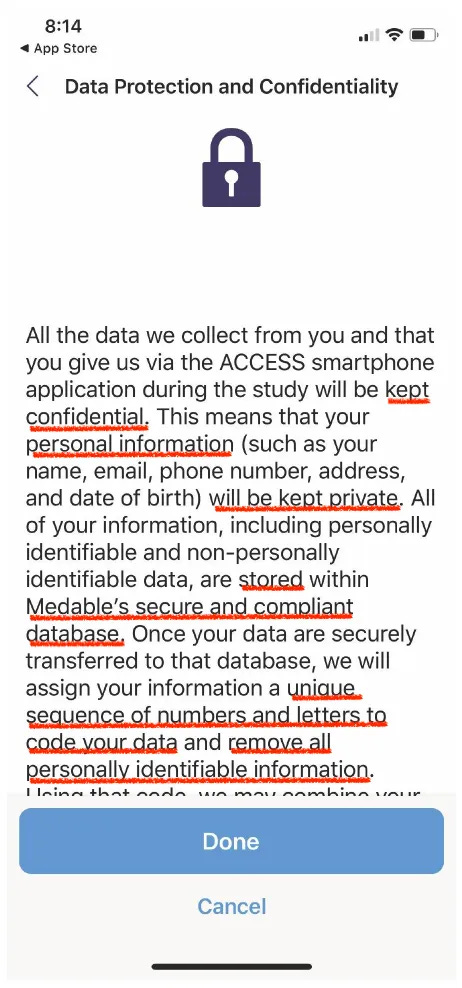
In the time of COVID-19 all the small print on Medable apps are archived in this Privacy Statement that was last modified on March 7 2022. The ACCESS App Privacy Statement was last modified on 26th July 2021 where Clause VI ‘We May Disclose Your Personal Information to Certain Outside Parties.’
In the time of COVID-19, in 2021 Oracle Health Sciences sponsors and presents a slideshow to discuss ‘Addressing Roadblocks in Decentralized Trials’ and it’s 5 challenges with representation from CRO’s: Medable UK, Bristol Myers Squibb, Decentralized Trials and Research Alliance, IQVIA Decentralized Trials & Novartis.


A 2024, ‘deep dive’ on Medable’s ‘partnership network’ includes companies like ORACLE, GSK, Syneos health and Cognizant.
3.6 The Heart Switch-eroo
In 2016, Dr Longmire’s Medium Article ‘Bridging the digital (health) divide’15 promotes the USC ‘Center for Body Computing’ with an announcement made in conjunction with Facebook’s Mark Zuckerberg $3B pledge to help end disease:
The Center for Body Computing, of which my company is a founding member, announced its Digital Health Access Initiative to provide smartphones/data plans to the underserved. Researchers were stifled because of regulations, complex development, and cost. Now, with Axon [by Medable that connects to Apple ResearchKit] health experts can easily build clinical study applications without a developer. We are 'Uberizing' clinical study apps. We want to see other companies intelligently re-think healthcare, too.
In 2014, this Forbes article on Dr Leslie Saxon (LS), renowned mentor to many sports warriors, including Longmire, and Executive Director of the USC Center for Body Computing and Chief of cardiovascular medicine at it’s Keck School, says that:
Dr Leslie Saxon wants to know what your heart rate is, arguing that Facebook should be given access to all of our medical data in order to help doctors better understand certain diseases through biometric data.
In 2006, Dr Saxon had made the ‘switch to digital health tracking’ by taking data wirelessly from patients' pacemakers and other devices with 308k digital patient interactions as of 2014. In 2012, USC Center for Body Computing ‘Wireless health’ was innovating under the slogans:
Your health is your life narrative.
We bring together 'techies' (engineers, technologists, physicians) with 'creatives' (designers, artists, Academy Award winners) to think about and create useful, innovative, scaleable, and often fun, solutions to help solve problems, and save lives.
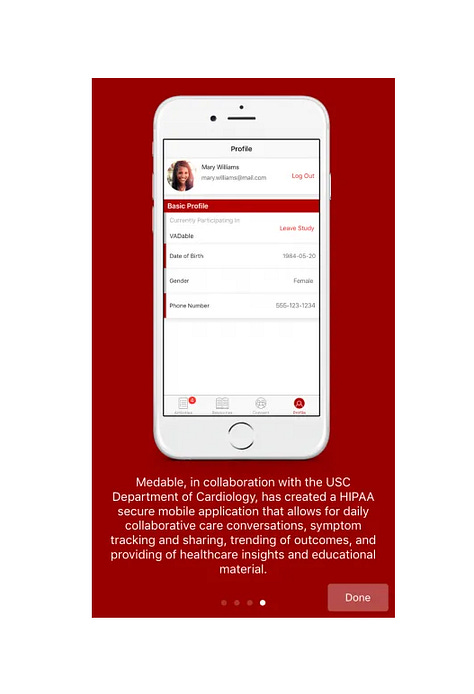
In 2016, USC Cardiology Dept launches Ventricular Assist Device ResearchKit app, VADable in partnership with Medable, to allow cardiac teams to collect real time data from patients with VAD devices in place from the pump itself, the wearers skin condition at the pump site and the ‘Health metrics’ monitored from your iPhone’s built in health metrics features — such as steps, calories burnt, sleep time (syncing with Health app). The VADable app and logo are illustrated in the ‘image gallery’ below with Dermtap/Medable apps. A 1:56 min ‘Medale Axon’ marketing video asks these Medable personnel and partners:
What would you study if you could connect directly with populations and patients anywhere on the earth any time of the day.. not just 5, 10 or even hundreds of patients but thousands and even Millions?





In the time of COVID-19 for the October 2020 annual ‘Body Computing’ event #USCBCC thanks their generous sponsors:
And in January 2021 ‘Sports Innovation Lab,’ Fluid Fans Podcast, Episode #41 ‘A Safe Return To Play with Dr. Leslie Saxon’ is asked to share her knowledge of the:
Cardiovascular health risks of COVID [and] safety protocols needed to get fans back in the stands..
04:14 Role?
Much of career spent in interventional cardiology in a field called cardiac electrophysiology, which among other things deals with the problem of sudden cardiac death in the athlete, you know, tragic kind of event..Started Body computing to optimise the athlete. It's all your medical record, your athlete record, your electronic medical record. We call it ‘Life care’.
18:00 Risk of Covid?
Starting to be guidelines that are emerging saying, look, if someone's not completely recovered or they had a significant infection, maybe make sure they don't, that have signs of cardiac inflammation or blood inflammation that could, lead to one of these adverse outcomes, particularly in collegiate sports.32:54 Perspective?
Health information, if it were [from] somebody all the time you protect it. [So] it can’t be weaponized.
In March 2021 Dr Saxon is interviewed by the ‘IEE Spectrum for Technology Insider’ for their article ‘Pro Sports COVID-19 Sensors Trace Rise of Ultra-Wideband Tech Bluetooth and UWB-based contact tracing’. In this interview, Dr Saxon was mostly concerned with the tech not solving some of the other issues brought up in their Body Computing paper related to if people are going to comply with digital contact tracing. The paper16 in question from October 2020 asks:
COVID-19 testing and infection surveillance: Is a combined digital contact-tracing and mass-testing solution feasible in the United States?
Summary: It will take time to vaccinate the entire population and vaccine hesitancy may hinder the vaccine’s ability to gain sufficient levels of protection for herd immunity. The Sooner we can understand how to create an effective COVID-19 surveillance and containment strategy, the sooner people can get back to work, kids can get back to school, economies can reopen, and supply chains can be reinstated. With mass testing, technology provides an opportunity to strengthen the fight against this virus and return to normalcy. It is imperative for the response to be unanimous and united to live freely again.
Sources of Funding: None.
Disclosures: All authors have no conflicts of interest to disclose.
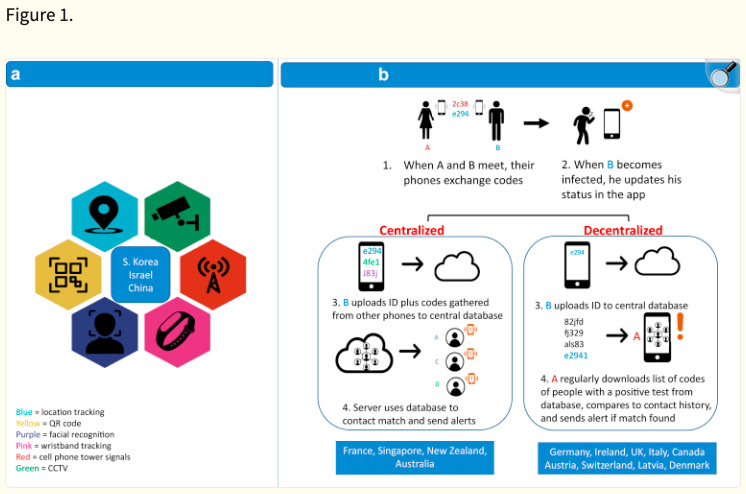
Figure 1. from the paper illustrates the more compliant ‘public’ health orientated countries contact tracing systems as either ‘centralised’ or ‘decentralised’.:
This October 2020 paper contains an Erratum17 from April 2021 with the appropriate ethics statements, provided by the Authors that were not included in the ‘Cardiovascular Digital Health Journal’ published version at the time. An example from Dr Saxon’s paper is:
Retrospective Study 8:
‘Utilizing electronic health data and machine learning for the prediction of 30-day unplanned readmission or all-cause mortality in heart failure’ [Cardiovascular Digital health Journal, 2020; 1 (2): 71-79] https://doi.org/10.1016/j.cvdhj.2020.07.004Ethics/guidelines clarification: The study was a retrospective chart review. Consent would be impossible or impracticable to obtain for such research so was waived by the research ethics committee. The study was conducted according to the principles of the Declaration of Helsinki.
4. Conclusion and Ascension
4.1 The Cyber Safety Net
In Feb 2024 Dr Saxon completed a 3 year term on the US Federal government Health Sector Coordinating Council (HSCC) Cybersecurity Working Group Executive Committee who released a 5-Year Plan that ensures by 2029 a:
Healthcare cybersecurity future state [in which] a cyber safety net [promotes].. cyber equity among under-resourced health organizations across the ecosystem.
The current US Federal Government HSCC 2025 membership list with 394 Private Sector Voting, 63 Non-Voting Advisors and 22 from Government includes Health Canada and the Alberta Health System! Private Sector Voters like GSK, Pfizer, Bayer, Google Health et al and even Ascension (Health System), MITRE and Medtronic!
1996 The physicians's guide to clinical research opportunities : how to create a rewarding business and professional relationship between your medical practice and the pharmaceutical industry by Heller, Matthew D., 1943- Boyle, James A., 1936-
Publisher: Los Angeles, CA : Practice Management Information Corp
https://archive.org/details/physicianssguide0000hell/page/n3/mode/2up
2020 March Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China
Qiurong Ruan, Kun Yang, Wenxia Wang, Lingyu Jiang and Jianxin Song
March 2020 Intensive Care Medicine 46(2)
https://web.archive.org/web/20250410011642/https://www.researchgate.net/publication/339661963_Clinical_predictors_of_mortality_due_to_COVID-19_based_on_an_analysis_of_data_of_150_patients_from_Wuhan_China
2005 Frishauf P. Medscape – The First 5 Years. MedGenMed. 2005 May 20;7(2):5. PMCID: PMC1681598. https://web.archive.org/web/20250210114705/https://pmc.ncbi.nlm.nih.gov/articles/PMC1681598/
2018 July Threats to oral polio vaccine acceptance in Somalia: Polling in an outbreak
Gillian K. SteelFisher a, Robert J. Blendon a b, Rustam Haydarov c, William Lodge II a, Hannah Caporello a, Sherine Guirguis d, Saumya Anand e, Julianne Birungi e 1, Matthew R. Williams f, Eran N. Ben-Porath g, Denise O'Reilly h, Christoph Sahm i
Vaccine Volume 36, Issue 31, 25 July 2018, Pages 4716-4724
https://www.sciencedirect.com/science/article/abs/pii/S0264410X18308144?via%3Dihub
aHarvard T.H. Chan School of Public Health, Boston, MA, USA
bJohn F. Kennedy School of Government, Cambridge, MA, USA
cUNICEF, Eastern and Southern Africa Regional Office, Nairobi, Kenya
dUNICEF, New York, NY, USA
eUNICEF, Somalia Country Office, Nairobi, Kenya
fIndependent Statistician, Reston, VA, USA
gSSRS, Media, PA, USA
hInterMedia, Washington, DC, USA
iOxford Research International, Oxford, UK
2021 - 2023 Blockchain for value creation in the healthcare sector
Rosanna Spanò a, Maurizio Massaro b, Silvia Iacuzzi c
Received 2 August 2021, Revised 8 November 2021, Accepted 12 December 2021, Available online 20 December 2021, Version of Record 23 January 2023
Technovation Volume 120
aDept of Economics, Management, Institutions, Federico II University of Naples, Naples, Italy
bDept of Management, Ca’ Foscari University of Venice, Venice, Italy
cDept of Economics and Statistics, University of Udine, Udine, Italy
[Silvia Iacuzzi is an Assistant Professor at Udine University. After completing a honours degree in Politics, Philosophy and Economics at the University of Oxford (England), she obtained her PhD from the University of Tübingen (Germany). She has spent the last 25 years working as a researcher, management and strategy consultant in Europe, the Middle East, Asia and Africa. Her research focuses on public sector accounting and management, looking at value creation, stakeholder engagement, performance measurement and management in particular for local government and healthcare organizations].
https://www.sciencedirect.com/science/article/abs/pii/S0166497221002212
2025 3rd March www.Medable.com Archive: ‘Medable Decentralized Clinical Trial Software (DCT and eCOA solutions)’
&
2014 24th Februrary www.Medable.com archive ‘Medable’
https://archive.is/www.medable.com/
2004 ‘Fifty Odd Years of EMP’ Dr. Conrad L. Longmire NBC Report Fall / Winter 2004 p 47
NBC Report is published semi-annually by United States Army Nuclear and Chemical Agency (USANCA) MISSION Provide nuclear & chemical technical expertise in support of all Army elements and to other US Government and NATO agencies as requested
https://www.futurescience.com/emp/NBC_Report_Fall_Winter04.pdf
2008 September Jonathan Longmire, Ph.D. Biographical Sketch
https://www.linkedin.com/in/longmire-jonathan-9045817/
2025 Longmire, Jon Co-Founder at Rendija Research LLC Los Alamos, New Mexico, US
Specialities Biosecurity, molecular biology, genomics, biodefense.
https://www.linkedin.com/in/longmire-jon-a54a0b14/
2011 Mobile phone-enabled control of medical care and handicapped assistance.
Liu, L., & Liu, J.
Expert Review of Medical Devices. 2011 Nov;8(6):757-68.
https://pubmed.ncbi.nlm.nih.gov/22029471/
2011 Biometrics on Mobile Phone by Shuo Wang and Jing Liu
Dept of Biomedical Engineering, School of Medicine, Tsinghua University P. R. China
Recent Application in Biometrics
https://api.intechopen.com/chapter/pdf-preview/17035
2013 M-HELP: a miniaturized total health examination system launched on a mobile phone platform
Yang Yu , Jingjing Li, Jing Liu
Telemed J E Health 2013 Nov;19(11):857-65
https://pubmed.ncbi.nlm.nih.gov/24050610/
2020 June 17 Meet Richard Lim, co-founder and Managing Director of GSR Ventures
By Steven Vator News
https://archive.is/vator.tv/2020-06-17-meet-richard-lim-co-founder-and-managing-director-of-gsr-ventures/
2017 June 9 From clinical care to continuous care Michelle Longmire 3 min read
https://archive.is/medium.com/@michellelongmire/synapse-f8046243520b
2016 September 27 Bridging the digital (health) divide Michelle Longmire 3 min read
https://archive.is/medium.com/@michellelongmire/bridging-the-digital-health-divide-c6ae6693a352
2020 Oct 2 COVID-19 testing and infection surveillance: Is a combined digital contact-tracing and mass-testing solution feasible in the United States?
Devin Skoll, Jennifer C Miller, Leslie A Saxon
USC Center for Body Computing, University of Southern California, Los Angeles, California
Cardiovasc Digit Health J
https://archive.is/pmc.ncbi.nlm.nih.gov/articles/PMC7531333/
2021 April ERRATUM Referred to by Erratum Cardiovascular Digital Health Journal, Volume 2, Issue 2, April 2021, Pages 150-151
https://archive.is/pmc.ncbi.nlm.nih.gov/articles/PMC8890336/