DIAlog AI: Part Three: Big Data Convergence with Farma Futures Forced by Forgiving or Forgetting? Does the Ascent of Our Avatar mean 24/7 Surveillance Tech Testing for Tailored Genetic Products?
Dial-a-Docs Dirty Dozen 7 to 9: "Data Analysis & Real World Interrogation Network” DARWIN = ALL our Real World Data harvested for Real World Evidence in Live Genomic Level Clinical Trials
Dial-a-docs 7a) ‘Data Analysis and Real World Interrogation Network’ DARWIN is a Big Data Task Force[d] Convergence. Where Real World Data is your medical records mined from all sources private, social and official. To Feed into Flexible Regulatory Processes for product cycle development.
2023 Jan DARWIN EU Members include co-chairman Emer Cooke, ‘European Pharmaceutical Industry Observer’ Almath Spooner (EFPIA, EuropaBio, EUCOPE) and ’EMA Project Team representative’ Peter Artlett.
Dr. Artlett via Nevernow continued from Part 2:
DARWIN EU is based on the principle of secondary use of healthcare data.
Access to databases containing information on real-world data, or data that is collected about a patient’s health or the delivery of care from a variety of sources other than clinical trials.
The analysis will generate real-world evidence which can then be used in regulatory decision making.
The ascendancy of real-world evidence complements the randomised controlled trial.
The whole point of randomisation is to deal with bias, consider it very carefully if real-world evidence is being used as one of the principal sources of evidence for efficacy.
2023 Regulatory Affairs Professionals Society: ‘Convergence: DARWIN EU real-world research network takes first steps’:1
Real-world data sources for DARWIN EU will include data on primary and specialist care, hospital care electronic health records, claims databases, disease registries, patient-reported outcomes, and drug prescription and dispensing data.
Real-world analyses generated by the network are expected to aid in regulatory decision making
Potential future health crisis, predict drug shortages and providing evidence for the repurposing of existing medicines, as well as monitoring the safety and effectiveness of vaccines and therapeutics.
Data Analysis and Real World Interrogation Network (DARWIN EU) :
The former HMA/EMA Big Data Task Force originally recommended developing DARWIN EU. The creation of DARWIN EU features in the EMA-HMA Big Data Steering Group workplan and the European medicines agencies network strategy to 2025.
May 2020: The Steering Group began its work in May 2020. It is co-chaired by Jesper Kjær, Director of Data Analytics Centre at the Danish Medicines Agency and Peter Arlett, Head of Data Analytics and Methods at EMA.
Aims to increase the utility of big data [real world data] in regulation, from data quality through study methods to assessment [real world evidence] and decision-making. It is patient-focused and guided by advances in science and technology.
Nov 2020: Nikolai Brun: The Timing is Now: Joint HMA EMA Big Data Task Force. From Dec 2019:
No 1# [of] Top-ten data recommendations: platform to access and analyse healthcare data across DARWIN-EU.
Big Data Task FORCE, Final Report December 2019:2
By delivering the vision of a regulatory system able to integrate Big Data into its assessment and decision making, [we] can support the development of innovated medicines, deliver life-saving treatments to patients more quickly and optimise the safe and effective use of medicines through measurement of a products performance on the market.
Dial-a-docs 7b) Real World Evidence from Real World Data is ‘We are Live!’ Clinical Trial-wise.
December 2022: Xavier Kurz, Data Analytics and Methods Taskforce, European Medicines Agency: 1st Dec 2022:3
By 2025 the use of Real-World Evidence will have been enabled and the value will have been established across the spectrum of regulatory use cases: Data Partners Phase 1 - 26 million Active Patients.
EHDS2 (European Health Data Science) Pilot phase: Use cases at a glance (WP9):
1) Surveillance of antimicrobial resistance;
2) Natural history of coagulopathy (blood clotting) related events in COVID-19 patients and risk factors;
3) Population uptake metrics: COVID-19 test positivity, vaccination and hospitalisation;
4) Comparing Nationwide Health trajectories to evaluate European Health Data interoperability: an application to cardiometabolic diseases;
5) Genomic data linked to health data, with a focus on Elixir.
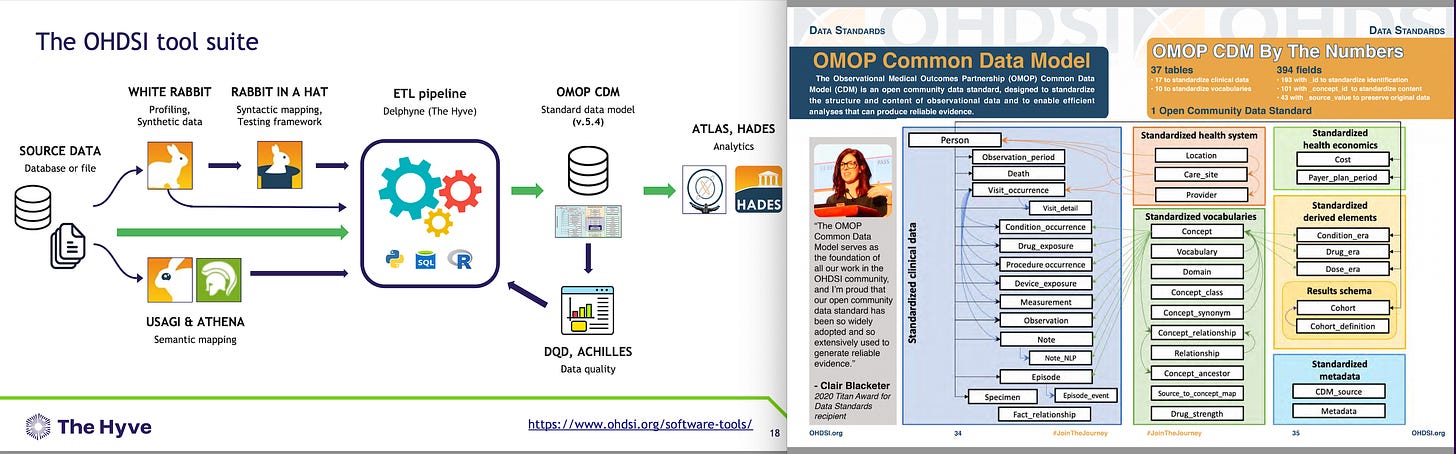
Dial-a-Docs 8a) Following the Data Leads to the White Rabbit. The White Rabbit is our Profiled Synthetic Data detailed in a Data Library called the ‘Book of Odyssey.’
October 2022: ELIXIR Bioinformatics Industry Forum: ‘Enabling Ecosystems for Machine Learning in the Life Sciences’4 Tue 11 October 2022, Henry Wellcome Auditorium, UK: hybrid event: Virtual participants will only access live stream and ask questions via SliDo Sofia Bazakou | The Hyve:
Use cases and lessons learned from facing challenges –The OHDSI approach to federated analysis: 2367 collaborators across 74 countries Patients, providers & researchers Health care systems, industry & government agencies
The OHDSI Tool Suite (Observational Health Sciences and Informatics, pronounced ‘Odyssey’) program is a multi-stakeholder, interdisciplinary collaborative to bring out the value of health data through large-scale analytics.
All our solutions are open-source: WHITE RABBIT = Profiling, Synthetic data, RABBIT IN A HAT = Syntactic mapping, Testing framework, USAGI & ATHENA = Semantic mapping, ETL (Extract, Transform & Load) pipeline = Delphyne (The Hyve), OMOP (Observational Medical Outcomes Partnership) CDM (Common Data Model) = Standard data model (v.5.4) DQD, ACHILLES = Data quality, ATLAS, HADES = ‘Analytics’.
September 2016: ‘The OHDSI Common Data Model and Extract, Transform & Load Tutorial’5 took place on September 24rd, 2016 during the 2016 OHDSI Symposium. Recordings were made possible by the generous support of Johnson & Johnson, the JKTG Foundation, and Pfizer.
ETL allows businesses to consolidate data from multiple databases and other sources into a single repository with data that has been properly formatted and qualified in preparation for analysis. This unified data repository allows for simplified access for analysis and additional processing. It also provides a single source of truth, ensuring that all enterprise data is consistent and up-to-date.
Chapter 4 The Common Data Model6 4.3.1 Running Example: Endometriosis:
Dial-a-Docs 8b) Following the White Rabbit written into the Book of Odyssey leads all the way back to the Watchdog Sentinel Initiative.
January 2011 - May 2009 ‘Observational Medical Outcomes Partnership’ Presentations7
’Pharmacovigilance and Risk Management Strategies - DIA panel:
Analysis for Health Care Data’8 presented by Thomas Scarnecchia Executive Director January 2011 DIA Home.org | National Institute of Health Foundation.
May 2009: ‘National Strategy for Monitoring Medical Product Safety’9 Patrick Ryan, GlaxoSmithKline on behalf of Paul Stang, PhD, Johnson & Johnson R&D and The Observational Medical Outcomes Partnership (OMOP) May 17th 2009.:
The Sentinel Initiative:
FDAAA SEC 905 establishes SENTINEL network of distributed observational databases (administrative claims and electronic health records) to monitor the effects of medicines post-approval process.
Dial-a-Docs 9) Following the White Rabbit to Hades Leads to the Ascent of our Digital Twin, ‘Simulacrum’. Our privacy-preserving synthetic profile to feed into the farm[a] live genomic trials for tailored products. That our farma infiltrated government watchdogs says is for our own good.
October 2022: Simulacrum | Health Data Insights (HDI) | Elixir | NHS Digital | biobank | CPRD | Genomics | UK Health Security Agency Roundtable Discussion:
Challenges with generation and adoption of privacy-preserving synthetic data:
Lora Frayling Data Scientist HDI: ‘Enabling Access to sensitive data’:10
Synthetic Data can be released for use in private research with minimal risk to patient privacy: Simulacrum is a synthetic version of datasets collected by NCRAS, NHS Digital Tumour diagnoses and chemotherapy treatments of 2.2M cancer patients in England (2013-2017) Version II coming later this year, including radiotherapy and genetic data (2016-2019).
UKRI Biotechnology and Biological Sciences Research Council (BBSRC) : Daniela Hensen | Technology development Oliver Huxley: BBSRC UKRI:11
Publishes review of data-intensive bioscience – UKRILINK leverage a 50% cash and/or in-kind contribution from the industry partner:
Digital Twins: Physical (Actuate: Sensor) Digital (Data Analytics: Insights).
Digital Twins: Watch this Space.
2022 Sep 19 ‘Convergence: DARWIN EU real-world research network takes first steps’
By Mary Ellen Schneider Regulatory News, PHOENIX AZ
http://web.archive.org/web/20220919154939/https://www.raps.org/news-and-articles/news-articles/2022/9/convergence-darwin-eu-real-world-research-network
2019 HMA-EMA Joint Big Data Taskforce Phase II report: ‘Evolving Data-Driven Regulation’
http://web.archive.org/web/20221008025057/https://www.ema.europa.eu/en/documents/other/hma-ema-joint-big-data-taskforce-phase-ii-report-evolving-data-driven-regulation_en.pdf
2022 Dec 1 ‘DARWIN EU – first experience and regulatory use cases EMA/HMA
Big Data Stakeholder Forum’ 2022 1st December 2022
Presented by Xavier Kurz Data Analytics and Methods Taskforce, European Medicines Agency
https://www.ema.europa.eu/en/documents/presentation/session-2-1-darwin-eu-r-first-experiences-and-regulatory-use-case-xavier-kurz_en.pdf
2022 Oct 11 ELIXIR Bioinformatics Industry Forum. Enabling Ecosystems for Machine Learning in the Life Sciences.
12:00 Session 2 (streamed online): Challenges and solutions in Machine Learning for the life sciences - Federated Learning and Synthetic Data
Moderator: Tim Beck, University of Leicester
2022 EBIF Agenda
’Session 2 Use cases and lessons learned from facing challenges –The OHDSI approach to federated analysis’ Speaker: Sofia Bazakou | The Hyve
https://elixir-europe.org/events/elixir-bioinformatics-industry-forum-enabling-ecosystems-machine-learning-life-sciences
2016 Sep 24 ‘Common Data Model and Extract, Transform & Load Tutorial’ and Resources
Observational Health Sciences and Informatics (OHDSI) Symposium
https://www.ohdsi.org/common-data-model-and-extract-transform-and-load-tutorial/
https://www.ohdsi.org/wp-content/uploads/2016/09/MAIN-OHDSI-Symposium-2016-Common-Data-Model-and-Extract-Transform-Load-Tutorial.pptx.pdf
2021 Jan 11 The Book of OHDSI - Observational Health Data Sciences and Informatics
Chapter 4 The Common Data Model - Chapter lead: Clair Blacketer
https://ohdsi.github.io/TheBookOfOhdsi/CommonDataModel
2017 Archvie ‘Observational Medical Outcomes Partnership’ Presentations
https://web.archive.org/web/20170709214104/http://omop.org/Presentations
2011 Jan 11 Pharmacovigilance and Risk Management Strategies - DIA panel:
Analysis for Health Care Data presented by Thomas Scarnecchia
Observational Medical Outcomes Partnerships
https://web.archive.org/web/20170710001251/http://omop.org/sites/default/files/Scarnecchia_DIA%20January%202011.pdf
2009 May 17 Midwest Biopharmaceutical Statistics Workshop Patrick Ryan
Observational Medical Outcomes Partnerships
https://web.archive.org/web/20170710002159/http://omop.org/sites/default/files/Midwest%20Biopharmaceutical%20Stats%20Workshop_May%2017_2009_Patrick%20Ryan.pdf
2022 Oct 11 ELIXIR Bioinformatics Industry Forum. Enabling Ecosystems for Machine Learning in the Life Sciences.
14:30 Round table discussions on Emerging issues in the application of Machine Learning to the Life Sciences (Auditorium | Franks & Steels Room)
Moderator: Vincent Zoete, University of Lausanne
2022 EBIF Agenda
5. Theme: Synthetic Data
’Roundtable Discussion: Challenges with generation and adoption of privacy-preserving synthetic data’
Presenter: Lora Frayling MSc, Data Scientist, Health Data Insight (HDI) NHS (Simulacrum)
https://elixir-europe.org/events/elixir-bioinformatics-industry-forum-enabling-ecosystems-machine-learning-life-sciences
2022 Oct 11 ELIXIR Bioinformatics Industry Forum. Enabling Ecosystems for Machine Learning in the Life Sciences.
12:00 Session 2 (streamed online): Challenges and solutions in Machine Learning for the life sciences - Federated Learning and Synthetic Data
Moderator: Tim Beck, University of Leicester
2022 EBIF Agenda
’Session 2 Setting the Scene –Artificial intelligence in the biosciences – a funder’s perspective’ Speaker: Daniela Hensen | BBSRC UKRI
https://elixir-europe.org/events/elixir-bioinformatics-industry-forum-enabling-ecosystems-machine-learning-life-sciences